Necessity Of Filtration In Ethylene Production
There are many ways chemical plants that rely on filtration can accomplish the desired reaction or separation. Whether it's by purifying feedstocks, removing emulsions from chemicals, filtration for process fluid reuse, or final production purification, the need for efficient filtrations systems applies to many applications. Chemical plant operators want to efficiently and reliably filter contaminants from a wide variety of process and product streams, which can cause a multitude of costly operational and fluid quality problems.
Ethylene Production (Hot Section)
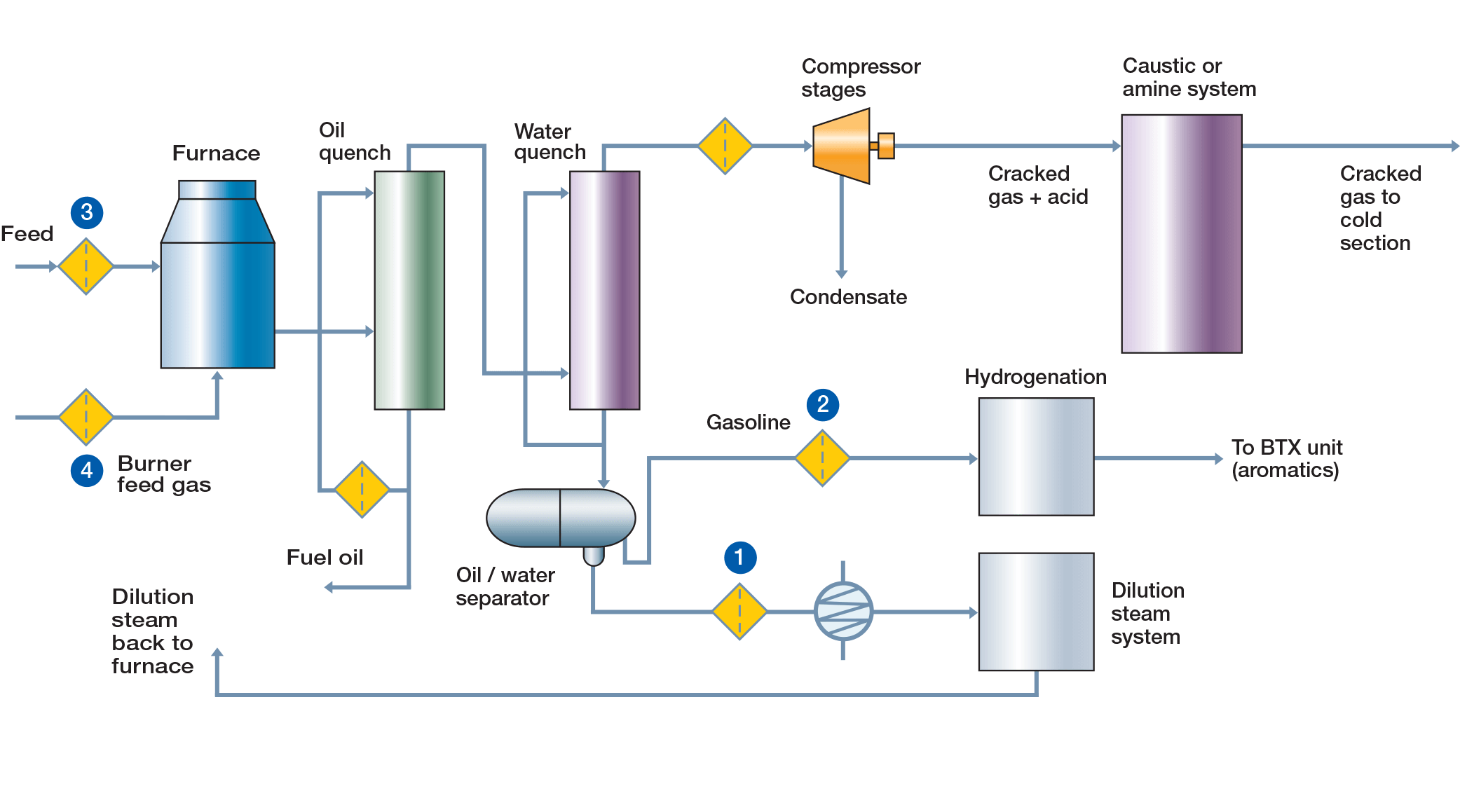
In the hot section, gas feedstocks (ethane, propane, blends) or liquid feedstocks (naphtha, gas oil) are cracked in a high temperature furnace (~800 °C). Dilution steam is introduced into the furnace to reduce the hydrocarbon partial pressure, thus favoring the production of ethylene and to reduce coke formation. The intense heat in the radiant section causes the hydrocarbon to undergo numerous chemical transformation reactions. The hot gas exits the radiant section and is quickly cooled to reduce the reactivity of effluent gas in exchangers and quenched to separate coke, solids and pyrolysis gasoline (pygas). It is then compressed and washed to remove acidic gases (CO2 and H2S). In the purification (cold) section, gases are dried and refrigerated via cold boxes to separate the various products (methane/hydrogen, ethylene, propylene, butadiene) by fractionation (distillation).
Coalescer technology addresses challenges in the ethylene production process by:
- High efficiency coalescer, usage results in guaranteed free pygas levels in quench water<20 ppm. Coalescer life is enhanced to about 1 year
- Effective removal of corrosion by products in feedstock feeds and if required, sodium in liquid feeds to furnaces <0.2 ppm
- Substantial removal of particulates and hydrocarbons in fuel/ cracked gas with surface treated SepraSol® Plus coalescer to improve burner efficiency
- Longer process run times at lower risk of encountering upset process conditions
Ethylene Production Challenges & Pall Solution
Challenge | Solution |
| Separate tar-like solids and stable emulsions of pygas and water to improve the quench water quality used in the dilution steam system (DSS) | Use of appropriate prefilters and process improvements to protect liquid/liquid coalescer in quench water applications. This will ensure long life of the prefilters and maximize coalescer performance. |
| Contaminants in the feed gas or liquid to furnaces must be removed to minimize coke formation | Removal of solids in gas and liquid feeds with highly efficient particulate filters. In some cases, removal of sodium in liquid feeds with proprietary liquid/liquid coalescers. |
| To improve furnace efficiency, burner tips must be protected from carbonaceous deposits | Use of surface treated liquid gas coalescer elements to remove hydrocarbons such as green oil from fuel gas to burners. |
Process Flow of Ethylene Production - Hot Section
Key Applications/Filter Recommendations For Ethylene Purification (other applications not shown)
Application | Pall Product | Advantages | Customer Benefits |
| Removal of pygas from quench water to make dilution steam. | For gas crackers, a combination of prefilters will typically include DFT Classic® + MCC1401E @10 μm + PhaseSepⓇ L/L coalescer. For liquid crackers, usually one prefilter @ 10 μm to protect coalescer. | Remove tar-like solids and break stable emulsion of pygas/ quench water, Reduce free pygas to <20 ppm in quench water. | The combination will ensure longer prefilter life and coalescer life - 1 year, resulting in savings on downstream maintenance cost (exchanger and steam generator)/ steam usage in strippers. It will reduce water treatment costs. |
| Removal of water from pygas before hydrogenation. | Prefilters such as MCC1401E or Coreless PPS @ 10 μm + PhaseSepⓇ L/L coalescer. | Break the emulsion of water/ pygas and ensure minimum water in product pygas. | Easier separation than in #1 above. Customer assured of on-spec pygas sent to hydrogenation reactor. |
| Removal of solids/ aqueous liquids from gas or liquid feeds to furnaces. | Filters for gas feeds are usually Coreless PPS @ 0.3 μm. For liquid feeds, Ultipleat® High Flow GF or MCC 1401E @ 10-20 μm as prefilters and if required, use PhaseSep L/L coalescer. | Remove solid iron sulfide/ oxide from feedstock with high efficiency filters and to remove sodium in water from liquid feeds with fluorocarbon coalescers. | Pall has extensive experience in removing solid and liquid contaminants in furnace feeds. Results in efficient furnace use with less decoking cycles at high throughput. |
| Protection of furnace burner tips. | SepraSol or SepraSol Plus L/G coalescer in fuel gas line. Medallion™ HP L/G coalescer is another option. | To mitigate fouling of burner tips. Premium surface treated or pleated Tier 2 L/G coalescer effectively removes solids and green oil in fuel gas to burners. | Customer benefits from significant improvement in furnace efficiency and less maintenance cost. |
| (Positions relate to flow diagram) | |||
Ethylene Production Hot Process Facts
- Usage: As a basic building block in the manufacture of ethylene glycol, polyethylene, polypropylene, polystyrene, polyester resin, polyvinyl chloride, etc.
- Form of final product: Gas with low boiling point.
- Feedstocks: Ethane, propane, ethane/propane, naphtha, gas oil.
- How it’s made: By cracking of gas or liquid feeds in high temperature furnaces, followed by quenching the cracked gas and obtaining various products by fractionation.
For more information about High Flow Filtration Technology, please see below.
For more information on improving the efficiency of your processes, contact our team of filtration experts.